Top 5 Things to Know About the New HIV Drug Fostemsavir
By:
- Dor Partosh, BPharm, PharmD Candidate
College of Pharmacy, Nova Southeastern University - Luis Urbina, PharmD Candidate
College of Pharmacy, Nova Southeastern University - Elizabeth Sherman, PharmD, AAHIVP
College of Pharmacy, Nova Southeastern University
South Florida, Southeast AIDS Education and Training Center
On July 2, 2020, the US Food and Drug Administration approved fostemsavir (Rukobia®), a first-in-class HIV-1 attachment inhibitor. Fostemsavir is a prodrug, which is hydrolyzed by alkaline phosphatase in the small intestine to the active moiety, temsavir. It binds directly to HIV-1 gp120, preventing initial viral attachment and entry into host CD4+ T cells. Fostemsavir’s mechanism of action is unique; other HIV-1 entry inhibitors work on different phases of entry into host CD4+ T cells. Fostemsavir is indicated, in combination with other antiretrovirals, for heavily treatment-experienced patients due to resistance, intolerance, or safety considerations. It has not been studied in treatment naïve patients, nor is it likely to be studied in this patient population. The recommended dosage of fostemsavir is one extended-release 600-mg tablet taken orally twice daily, with or without food. It should not be chewed, crushed, or split because of the extended release nature of the tablet.1-3 Summarized below are the top 5 things for all prescribers to know before prescribing fostemsavir.
Fostemsavir is reserved for highly-treatment experienced patients with limited antiretroviral treatment options, as demonstrated in the BRIGHTE trial[4].
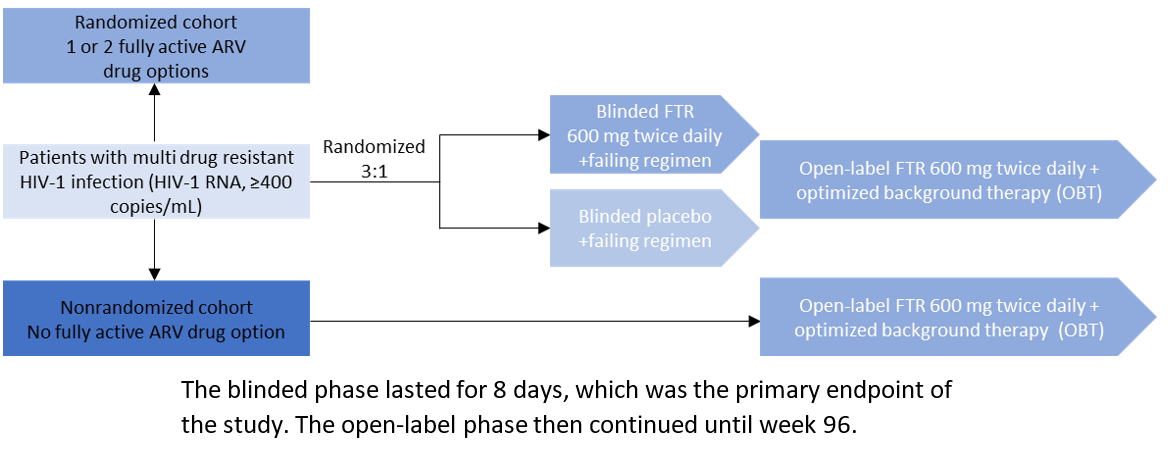
The BRIGHTE study was the major clinical trial that led to fostemsavir’s approval. The trial enrolled patients from 108 sites in 23 countries on six continents. Eligible patients were adults (≥18 years of age) who had undergone multiple treatments for HIV-1 infection. All patients had failure of their current antiretroviral regimen (as defined by HIV-1 RNA ≥400 copies per milliliter), and no viable antiretroviral combination therapy was available to them because of exhaustion of at least four of six antiretroviral classes (nucleoside reverse-transcriptase inhibitors, non–nucleoside reverse-transcriptase inhibitors, integrase inhibitors, protease inhibitors, CCR5 antagonists, and entry inhibitors) due to resistance, intolerability, contraindication, or safety concerns.4 The study methodology is summarized in Figure 1 below. In the study, 371 patients were treated: 272 in the randomized cohort and 99 in the nonrandomized cohort. In the randomized cohort, virologic response (HIV-1 RNA <40 copies/mL) was evaluated at weeks 24 (53%) and week 96 (60%). In the non-randomized cohort, virologic response was 37% at both week 24 and 96.1
Figure 1: BRIGHTE Trial Design
Fostemsavir has a unique side effect profile and may cause certain lab abnormalities.
The most common side effect when taking fostemsavir is nausea (10%). Patients should be counseled on this potential side effect. Certain laboratory abnormalities were observed in the clinical trials with fostemsavir, including an increase in serum creatinine of > 1.8 x ULN or 1.5 x baseline (19% of patients in the randomized cohort) and an increase in direct bilirubin >ULN (7% of patients in the randomized cohort).1 These abnormalities were more prevalent in patients with related comorbidities such as chronic kidney disease and diabetes for serum creatinine elevations, and sepsis or viral hepatitis for direct bilirubin elevations. A link for these occurrences has not been established and they were mostly transient in nature. These abnormalities often resolved with continuation of treatment and did not have clinical significance. Additionally, elevations in hepatic transaminases have been seen in the clinical studies of fostemsavir, and were seen in a greater proportion of patients with hepatitis B or hepatitis C co-infection. Some of these elevations in transaminases were consistent with hepatitis B reactivation. Co-infected patients starting fostemsavir should be appropriately maintained on effective therapy for hepatitis B.
Fostemsavir is metabolized by the cytochrome P450 enzyme system and has many clinically significant drug interactions with other medications.
Strong P450 inducers may lower fostemsavir drug concentrations, and their use is contraindicated with fostemsavir. These include androgen receptor inhibitors (enzalutamide), anticonvulsants (carbamazepine, phenytoin), antineoplastics (mitotane), herbal products (St. John’s wort), and antimycobacterials (rifampin). Rifabutin may safely be coadministered with fostemsavir and may be a suitable alternative if drug interactions preclude the use of rifampin.1
Importantly, when fostemsavir is co-administered with ethinyl estradiol, contained in some oral contraceptives, the concentration of ethinyl estradiol is increased by ~40% in both AUC and Cmax. Therefore, the max daily dose of EE should not exceed 30 µg and caution is advised in patients with other risk factors for thromboembolic events. 5
Finally, fostemsavir does not have clinically significant drug interactions with other antiretroviral agents and may safely be coadministered with any currently available anti-retroviral regimen. 5
Fostemsavir does not require a hepatic or renal dose adjustment, including dialysis.
Fostemsavir does not require renal or hepatic dose adjustments. Fostemsavir has been studied in patients with normal renal function (eGFR> 90 mL/min/1.732), mildly decreased renal function (eGFR 89 – 60 mL/min/1.732), moderately decreased renal function (eGFR <60 – 30 mL/min/1.732), severely decreased renal function (eGFR <30 mL/min/1.732 not on hemodialysis) and end-stage renal dysfunction (eGFR <30 mL/min/1.732 on hemodialysis). For each of the stages the impact on fostemsavir Cmax was not clinically significant6. No dosage adjustment is required for patients with renal impairment or those on dialysis. Similarly, fostemsavir has been studied in patients with hepatic impairment (Child Pugh Score A, B, and C) and no dose adjustment is required in patients with mild, moderate or severe hepatic impairment. 1
Fostemsavir has no in-vivo cross-resistance with other antiretrovirals, including other entry inhibitors.
The antiviral activity of temsavir is not antagonistic in cell culture when combined with other entry inhibitors, including ibalizumab, maraviroc, or enfuvirtide. Prescribers may safely prescribe more than one entry inhibitor for patients. However, the entry inhibitors fostemsavir, ibalizumab, and maraviroc may all develop resistance in gp120. Nonetheless, there is no in-vivo evidence of cross-resistance. Viruses resistant to the gp41 fusion inhibitor enfuvirtide retain susceptibility to temsavir.1
References
- Rukobia [package insert]. Research Triangle Park, NC: GlaxoSmithKline; 2020.
- Moore K, Magee M, Ackerman P, et al. Pharmacokinetics of temsavir, the active moiety of the prodrug fostemsavir, in subjects with hepatic impairment. IDWeekTM 2017, San Diego, CA, USA. October 4-8, 2017: Abstract 1390.
- Lagishetty C, Moore K, Ackerman P, et al. Effects of temsavir, active moiety of antiretroviral agent fostemsavir, on QT interval: Results from a phase I study and an exposure–response analysis. Clin Transl Sci. 2020;13(4):769-776. doi:10.1111/cts.12763
- Kozal M, Aberg J, Pialoux G, et al. Fostemsavir in adults with multidrug-resistant HIV-1 infection. N Engl J Med. 2020;382(13):1232-1243.
- Moore K, Magee M, Ackerman P, et al. Fostemsavir drug-drug interaction profile, an attachment inhibitor and oral prodrug of temsavir, for heavily treatment-experienced HIV-1 infected patients. IDWeekTM 2019, Washington, DC, USA. October 2-6, 2019: Abstract 2500.
- Moore K, Magee M, Ackerman P, et al. Impact of mild, moderate and severe renal impairment and hemodialysis on temsavir pharmacokinetics following oral administration of fostemsavir, an attachment inhibitor for heavily treatment experienced HIV-1 infected patients. HIV Drug Therapy Glasgow 2019, Glasgow, UK. October 28-31, 2018: Abstract P261.