
DING DING DING! Get to know the 2 new fighters that have entered the ring against pneumococcal disease: PCV15 and PCV20.
By: Stephanie Suarez, PharmD Candidate1, Elguis Perez, PharmD Candidate1, Elizabeth Sherman, PharmD1,2
- College of Pharmacy, Nova Southeastern University
- South Florida, Southeast AIDS Education and Training Center
Background
The Advisory Committee on Immunization Practices (ACIP) recommended two new pneumococcal vaccination regimens on October 20, 2021, one dose of pneumococcal conjugate vaccine PCV15 (Vaxneuvance) followed by a dose of pneumococcal polysaccharide vaccine PPSV23 (≥1 year later) or one dose of PCV20 (Prevnar20), in adults ≥65 years of age or those 19-64 years of age with increased risk factors for pneumococcal disease.1 Prior to these updates, the available vaccines were limited to PCV13 and PPSV23. Centers for Disease Control and Prevention (CDC) recommendations from 2019 consisted of only PCV13 and PPSV23 for younger adults with a higher risk of having pneumococcal disease and adults ≥ 65 years of age. Please see Table 1 for a comparison between the 2019 and 2022 CDC pneumococcal vaccine guidelines based on the population being treated. HIV care providers should be familiar with these updated vaccination regimens, as they offer a more streamlined approach for pneumonia prevention in a patient population at increased risk.
On September 7, 2022 the National Institutes of Health, the CDC, and the HIV Medicine Association of the Infectious Diseases Society of America Panel released updated opportunistic infection guidelines for people with HIV. These guidelines are consistent with 2022 CDC pneumococcal vaccination recommendations. Based on the updates to the opportunistic infections guidelines, patients with CD4 ≥200 cells/mm3 should receive a dose of PPSV23 at least 8 weeks after PCV15. On the other hand, patients with CD4 <200 cells/mm3 (and preferably HIV RNA <100,000 copies/mL) could be offered PPSV23 after receiving PCV15 however PPSV23 should be deferred until after the CD4 count increases to >200 while on ART.2
PCV15 in a nutshell
PCV15 was FDA approved in July 2021. In addition to the serotypes included in PCV13, PCV15 also contains serotypes 22F and 33F. Healthy adults ≥50 years of age, adults with HIV infections ≥18 years of age, Native American adults between 18 and 49 years of age, or adults with ≥1 risk factors for pneumococcal disease (i.e., chronic heart, liver or lung disease, diabetes melitus, HIV infection, leukemia, lymphoma, solid organ transplants, alcoholics, cigarette smokers, renal failure, sickle cell, Hodgkins, cochlear implants, cerebral spinal fluid leak, nephrotic syndrome, iatrogenic immunosuppression, generalized malignancies, and congenital or acquired immunodeficiency or asplenia) were all evaluated in clinical trials for the immunogenicity and safety of PCV15. One study included 302 patients with HIV infection. One of the phase 3 trials of adults ≥ 50 years of age showed PCV15 was noninferior to PCV13.3 Most frequent adverse events that occurred were injection site reactions, fatigue, and myalgia. Safety wise, serious adverse events (SAEs) with PCV15 occurred in 2.5% of the patients in trials that included patients 18 years of age or older.
PCV20 in a nutshell
PCV20 was FDA approved in June 2021. PCV20 has multiple pneumococcal polysaccharide serotypes (8, 10A, 11A, 12F, 15B, 22F, 33F), including those in PCV13. Most frequent adverse events that occurred in these patients were muscle pain, injection site reactions, fatigue, headache, and joint pain. Safety trials for PCV20 showed that SAEs occur in 1.5% of recipients. In trials of PCV20 compared to PCV13 and PPSV23 among adults 60-64 years of age and adults ≥ 18 years of age, PCV20 has demonstrated noninferiority.4 In these studies, adults included were: ones with stable medical conditions, but none included adults with immunocompromising conditions.
Administration Timing
For a patient who has an unknown vaccination history or has never received a pneumococcal vaccine they can either be given one dose of PCV20 and be done or they can be given a dose of PCV15 followed by one dose of PPSV23 at least 1 year later. However, 8 weeks apart can be considered for PCV15 and PPSV23 if the patient has an immunocompromising condition (including HIV), cochlear implants or cerebrospinal fluid leakage, please see Figure 1 on administration timing. If the patient already received a dose of PPSV23 and no other conjugate vaccine then they can be given PCV15 or PCV20 at least 1 year after PPSV23, please see Figure 2 on administration timing. Patients do not need to be re-vaccinated once they are ≥ 65 years of age.5 As per the MMWR and HIV guidelines: patients who have HIV infection and have previously received only PCV13 should receive PPSV23 at least 8 weeks later or people with HIV who have received PCV13 and PPSV23 should receive a booster PPSV23 at least 5 years after the first dose (see Table 1).5
Cost Comparison
Health outcomes and costs were shown in economic models for PCV15 and PCV20 as compared with previous recommendations.1 PCV20 alone for adults aged ≥65 years of age and for 19-64 years of age with an underlying risk factor showed $39,000 and $11,000 to $292,000 per quality-adjusted life-year (QALY) gained respectively. While PCV15 in series with PPSV23 for adults 65 years of age or older and for those 19 to 64 years of age with underlying conditions showed $282,000 and $250,000 to $656,000 per QALY gained respectively.
Summary
In conclusion, the 2022 guidelines recommend two new pneumococcal vaccine regimens. Those being one dose of PCV20 alone or one dose of PCV15 followed at least one year later by one dose of PPSV23. These vaccines show efficacy in adults ≥65 years of age with or without immunocompromised conditions and adults 19 to 64 years of age with underlying risk factors. If a patient previously administered PCV13, they should complete PPSV23 in series.6 Both vaccine regimens showed minimal adverse events; however, if adverse events do occur they should be reported to the vaccine adverse event reporting system (VAERS). PCV15 and PCV20 should both be stored refrigerated in 36° F to 46° F and should not be frozen. Co-administration of the pneumococcal vaccine with the flu vaccine (Fluarix/Fluad) has been demonstrated to be safe and immunogenic.1
| Population | 2019 Guidelines6 | 2022 Guidelines1 |
|---|---|---|
| 19-64 years of age with underlying risk* |
IMMUNOCOMPETENT 1 dose of PPSV23£ IMMUNOCOMPROMISED€ 1 dose of PCV13 followed by 1 dose of PPSV23 ≥8 weeks later and a 2nd dose of PPSV23 ≥5 years later |
1 dose of PCV20 or 1 dose of PCV15 followed by a dose of PPSV23 ≥ 1 year later** |
| ≥ 65 years of age |
NOT IMMUNOCOMPROMISED IMMUNOCOMPETENT£ 1 dose of PPSV23; if PCV13 has been given, then give PPSV23 ≥1 year after PCV13 and ≥5 years after any PPSV23 <65 years IMMUNOCOMPROMISED€ 1 dose of PCV13 if no previous PCV13 vaccination; 1 dose of PPSV23 ≥8 weeks after PCV13 and ≥5 years after any PPSV23 at <65 years |
1 dose of PCV20 or 1 dose of PCV15 followed by a dose of PPSV23 ≥ 1 year later*** |
*Underlying conditions: Alcoholism, chronic heart disease, chronic liver disease, chronic lung disease, cigarette smoking, diabetes mellitus, cochlear implant, CSF leak, congenital or acquired asplenia, sickle cell disease, chronic renal failure, congenital or acquired immunodeficiencies, generalized malignancy, HIV infection, Hodgkin disease, latrogenic immunosuppression, leukemia, lymphoma, multiple myeloma, nephrotic syndrome, solid organ transplant
£Immunocompetent conditions: Alcoholism, chronic heart disease, chronic liver disease, chronic lung disease, cigarette smoking, diabetes mellitus
$Immunocompetent conditions: Cochlear implant and CSF leakage
€Immunocompromised conditions: congenital or acquired asplenia, sickle cell disease, chronic renal failure, congenital or acquired immunodeficiencies, generalized malignancy, HIV infection, Hodgkin disease, latrogenic immunosuppression, leukemia, lymphoma, multiple myeloma, nephrotic syndrome, solid organ transplant
**Immunocompromised conditions (including HIV), CSF leak or cochlear implants might benefit from shorter intervals (≥8 weeks)
***Immunocompromised conditions, CSF leak or cochlear implants might benefit from shorter intervals. No additional listed pneumococcal vaccine required
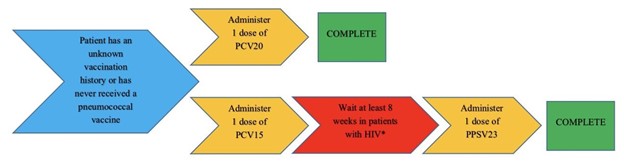
Figure 1: Vaccine timing for patients with HIV with unknown vaccine history or have never received a pneumococcal vaccine5
*Patients with CD4 ≥200 cells/mm3 should receive a dose of PPSV23 and patients with CD4 <200 cells/mm3 can also be offered PPSV23 especially if their HIV RNA <100,000 copies/mL2
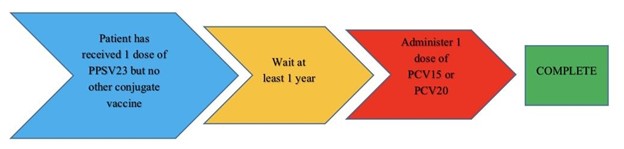
Figure 2: Vaccine timing for patients with HIV that already received PPSV235

Figure 3:Vaccine timing for people with HIV who previously received PCV132

Figure 4:Vaccine timing for people with HIV who have received PCV13 & PPSV232
References:
- Kobayashi M, et al. Use of 15-valent pneumococcal conjugate vaccine and 20-valent pneumococcal conjugate vaccine among U.S. adults: Updated recommendations of the Advisory Committee on Immunization Practices — United States, 2022. MMWR Morbidity and Mortality Weekly Report. 2022;71(4):109-117. doi:10.15585/mmwr.mm7104a1
- Immunizations for preventable diseases in adults and adolescents living with HIV: NIH. Immunizations for Preventable Diseases in Adults and Adolescents Living with HIV | NIH. https://clinicalinfo.hiv.gov/en/guidelines/hiv-clinical-guidelines-adult-and-adolescent-opportunistic-infections/immunizations?view=full. Published September 7, 2022. Accessed September 12, 2022.
- Platt HL, et al. A phase 3 trial of safety, tolerability, and immunogenicity of V114, 15-valent pneumococcal conjugate vaccine, compared with 13-valent pneumococcal conjugate vaccine in adults 50 years of age and older (PNEU-AGE). Vaccine 2022;40:162–72. https://doi.org/10.1016/j.vaccine.2021.08.049external icon PMID:34507861
- Essink B, et al. 3. Phase 3 pivotal evaluation of 20-valent pneumococcal conjugate vaccine (PCV20) safety, tolerability, and immunologic noninferiority in participants 18 years and older. Open Forum Infect Dis 2020;7(Supplement_1):S2. https://doi.org/10.1093/ofid/ofaa417.002
- Pneumococcal vaccine timing for adults – centers for disease control . https://www.cdc.gov/vaccines/vpd/pneumo/downloads/pneumo-vaccine-timing.pdf. Published April 1, 2022. Accessed September 6, 2022.
- Matanock A, et al. Use of 13-valent pneumococcal conjugate vaccine and 23-valent pneumococcal polysaccharide vaccine among adults aged ≥65 years: Updated recommendations of the Advisory Committee on Immunization Practices. MMWR Morbidity and Mortality Weekly Report. 2019;68(46):1069-1075. doi:10.15585/mmwr.mm6846a5
