“B” Smart and Find out the Latest about the Hepatitis B Vaccine in Patients with HIV
By:
- Jyddu Camauta , BPharm, Pharm D Candidate
College of Pharmacy, Nova Southeastern University
- Carina Diaz, Pharm D Candidate
College of Pharmacy, Nova Southeastern University
- Elizabeth Sherman, PharmD, AAHIVP
College of Pharmacy, Nova Southeastern University
South Florida, Southeast AIDS Education and Training Center
Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV were updated on September 7, 2022, in an effort to improve hepatitis B virus (HBV) vaccine response rates in patients with HIV. This population is at higher risk of acquiring HBV and less likely to produce HBV immunity during acute infection. Compared with patients who are only infected with HBV, patients with HIV and HBV coinfection have a higher risk of developing chronic HBV infection, cirrhosis, and end-stage liver disease. 1 Therefore, it is imperative to prevent HBV infection in patients with HIV.
All patients should be tested for hepatitis B surface antigen (HBsAg), hepatitis B surface antibody (anti-HBs), and antibody to hepatitis B core antigen (anti-HBc). In general, HBV vaccination is recommended if the patient was not previously exposed to HBV and this can be confirmed if testing results are HBsAg (-), anti-HBs (-), and anti-HBc (-). It is important to highlight that patients with CD4 cell counts ≥350 cells/microL and undetectable HIV RNA are more likely to develop immunity after vaccination. However, vaccination should not be delayed if patients have a low CD4 cell count, because there are cases where patients with CD4 cell count <350 cells/microL have developed immunity after vaccination (AII). Unfortunately, vaccine response rates in this population are lower than in patients that are not coinfected with HIV due to the recipient’s overall health condition. There are currently three FDA-approved single antigen recombinant HBV vaccines; Recombivax-HB, Engerix-B, Heplisav-B, and a combined hepatitis A-hepatitis B vaccine (Twinrix). 2
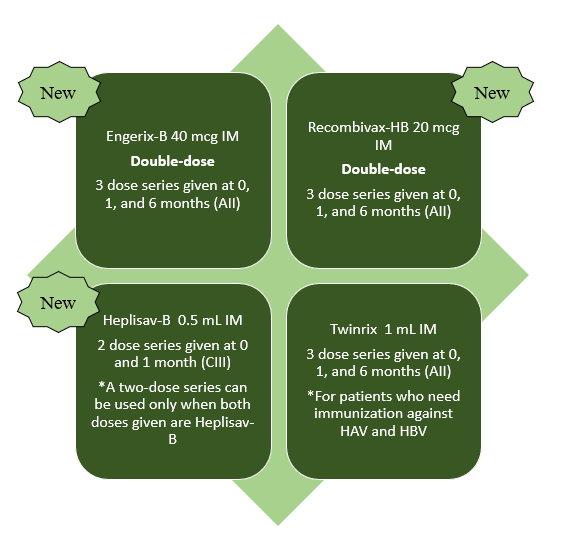
Recent guideline updates now recommend to administer a double-dose of Recombivax-HB or Engerix-B and include as an alternative the recombinant HBsAg vaccine conjugated to CpG 1018 (Heplisav-B). This new recommendation to use a double dose HBV vaccine is based on the outcomes of a recent meta-analysis of 10 studies that included a total of 970 patients with HIV.2 Results showed that a double dose of the hepatitis B vaccine was more effective than a single dose with odds ratio of 1.76 (95% CI: 1.36–2.29) and 2.28 (95% CI: 1.73–3.01) measured at 4–6 weeks and >12 months after completion of vaccination, respectively (AII).3 There is an ongoing study of the safety and efficacy of Heplisav-B in patients with HIV. A two dose Heplisav-B series was superior to a 3 dose series of standard dose Engerix-B in four randomized controlled trials that were conducted in healthy patients and patients with type 2 diabetes mellitus.2 In the largest trial, 8374 patients were randomized and 961 patients in the per-protocol population had T2DM. Results of this trial showed a 95 % protection rate with Heplisav-B versus an 81% protection rate for Engerix-B.4 Figure 1 shows the dosing and schedule for HBV vaccine in adult patients with HIV not on hemodialysis
Figure 1: Dosing and Schedule for Hepatitis B Vaccine in Adult Patients with HIV not on hemodialysis
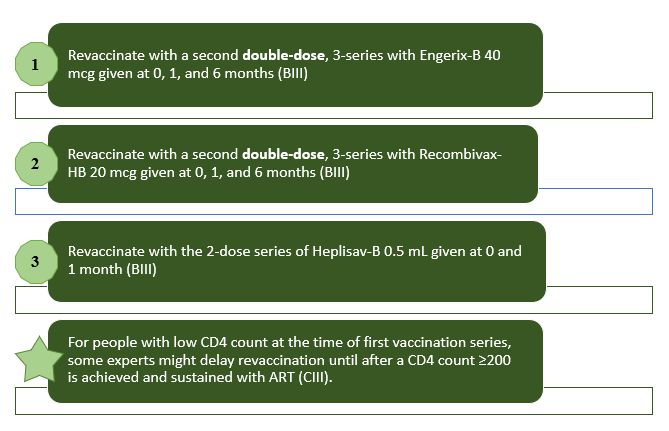
Once patients have received a complete HBV vaccination series, antibody testing is very important, and it should be done 4 weeks post-vaccination. A patient is considered to have developed protection against HBV if titer results are at least 10 mIU/mL. Patients with titers less than 10 mIU/mL after vaccination are nonresponders and there are a few options to consider.5 Figure 2 shows the dosing options and schedule for HBV vaccine in adult patients with HIV who are non-responders.
Figure 2: Dosing Options and Schedule for HBV Vaccine in Adult Patients with HIV who are Non-responders (anti-HBs titers <10 mIU/mL)
In summary, hepatitis B testing should be done in all patients with HIV and vaccination is recommended for those without evidence of protective immunity against HBV. Based on recent guidelines updates, a double-dose of the recombinant hepatitis B vaccine is recommended in order to improve vaccine response rates in this population.
References:
- Prevention of hepatitis B virus infection in adults with HIV. Up to date. Wolters Kluwer. https://online.uptodate.com. Updated November 19, 2021. Accessed November 10, 2022
- Panel on Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV. National Institutes of Health, Centers for Disease Control and Prevention, HIV Medicine Association, and Infectious Diseases Society of America. Available at https://clinicalinfo.hiv.gov/en/guidelines/adult-and-adolescent-opportunistic-infection. Accessed November 28, 2022
- Lee JH, Hong S, Im JH, Lee JS, Baek JH, Kwon HY. Systematic review and meta-analysis of immune response of double dose of hepatitis B vaccination in HIV-infected patients. Vaccine. 2020;38(24):3995-4000. doi:10.1016/j.vaccine.2020.04.022
- Jackson S, Lentino J, Kopp J, et al. Immunogenicity of a two-dose investigational hepatitis B vaccine, HBsAg-1018, using a toll-like receptor 9 agonist adjuvant compared with a licensed hepatitis B vaccine in adults.Vaccine. 2018;36(5):668-674. doi:10.1016/j.vaccine.2017.12.038
- Lesson 4. Immunizations in adults. National HIV Curriculum. https://www.hiv.uw.edu. Accessed November 10, 2022