A Lazy Person’s Guide to Latent Tuberculosis Treatment in Patients with HIV
By:
- Chelsey Axelrod, PharmD Candidate
College of Pharmacy, Nova Southeastern University - Haithuy Pham, PharmD Candidate
College of Pharmacy, Nova Southeastern University - Elizabeth Sherman, PharmD, AAHIVP
College of Pharmacy, Nova Southeastern University
South Florida, Southeast AIDS Education and Training Center
Tuberculosis (TB), caused by the bacterium Mycobacterium tuberculosis, is the leading cause of death from infectious disease worldwide, resulting in approximately 10 million infections and 1.5 million deaths annually. A person who is infected with TB without active symptoms is considered to have latent tuberculosis infection (LTBI). The estimated annual risk for active TB disease among persons with LTBI is about five percent, but it is three to twelve times greater for persons with HIV than for those without HIV. Therefore, treatment of LTBI in patients with HIV is of utmost importance. Prior to September 2019, the Guidelines for the Prevention and Treatment of Opportunistic Infections in Adults and Adolescents with HIV, recommended isoniazid for 9 months as the preferred regimen, and rifampin for 6 months as an alternative treatment.1 The guidelines have since been updated. This article reviews important updates to the LTBI section, including the use of newer short-course regimens, in an easy-to-read question and answer format.
Which patients with HIV should receive LTBI treatment?
- Those with a positive screening test for LTBI without evidence of active TB disease and no prior history of treatment for active disease or LTBI, or
- Those in close contact with any person with infectious TB, regardless of their TB screening test results
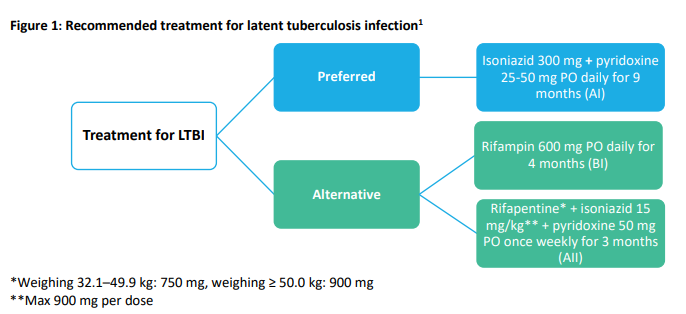
What are the options for treating LTBI in patients with HIV? (Figure 1)
- Isoniazid for 9 months (9H) is still the preferred regimen
- Rifampin daily for four months (4R) is still an alternative treatment
- NEW: Rifapentine plus isoniazid once weekly for 3 months (3HP) is now an alternative therapy
What are the studies supporting the addition of 3HP as an LTBI treatment option in patients with HIV?
- The PREVENT TB trial showed 3HP given was as equally effective and as well-tolerated as 9H in persons with HIV. Participants had not received HIV-1 protease inhibitors (PIs) and non-nucleoside reverse transcriptase inhibitor (NNRTI)-based antiretroviral therapy (ART) within 90 days of enrollment. A majority (~80%) of the participants had CD4 counts > 350 cells/microL.2
- An open-label, randomized trial found that there was no significant difference in efficacy between 3HP and 9H in persons with HIV. Participants were not receiving ART during the study and the mean CD4 count was 484 cells/microL.3
- A few pharmacokinetic and pharmacodynamic studies in HIV-infected persons receiving rifapentine with efavirenz or raltegravir-based ART showed that rifapentine had a minimal effect on ART concentrations.4,5,6,7
What are the clinically significant ART drug interactions when treating LTBI? (Table 1)
- 9H: No clinically significant drug interactions with ART.
- 4R: Contraindicated with all PIs, most NNRTIs, and a few integrase strand transfer inhibitors. Some antiretrovirals (ARVs) require dose adjustments.
- 3HP: Most ARVs are contraindicated, with the exception of raltegravir, efavirenz, and some nucleoside reverse transcriptase inhibitors.
It is essential to assess for drug-drug interactions between LTBI treatment, ART, and any other prescription and overthe-counter (OTC) medications prior to initiating treatment in patients with HIV. The assessment should also consider the pros and cons of the LTBI treatment options (Table 2). LTBI treatment and ART both contribute independently to lowering the risk of TB disease. Using both drug therapies is recommended for persons with HIV and LTBI. For patients exposed to drug-resistant TB, guidelines recommended that a consultation with experts or with public health authorities should take place prior to selection of a regimen for LTBI treatment. In conclusion, healthcare providers should continue to stay vigilant in screening and treating patients with LTBI.1
Table 1: Significant drug interactions between antiretroviral therapy and latent tuberculosis infection8 |
|||
| LTBI Treatment | ARV | Effect on Drug Concentrations | Recommendations |
| Isoniazid | All ARVs | ↔ ARV concentrations | No dose adjustment needed |
| Rifampin | TAF | TAF AUC ↓ 56% TFV AUC ↓ 53% |
Do NOT co-administer |
| DTG | DTG AUC ↓ 54% | Dosing of DTG: 50 mg twice daily | |
| RAL | RAL AUC ↓ 40% | Dosing of RAL: 800 mg twice daily | |
| MVC | MVC AUC ↓ 63% | Dosing of MVC: 600 mg twice daily | |
| EFV | EFV AUC ↓ 26% | Dosing of EFV: Do NOT use EFV 400 mg Standard (600 mg) if < 50 kg, 800 mg if ≥ 50 kg |
|
| All PIs, DOR, ETR, RPV, BIC, or EVG/c |
All AUC ↓ ≥ 75% | Do NOT co-administer | |
| Rifapentine | All other ARVs | No dose adjustment needed | |
| TAF | ↓ TAF possible | Do NOT co-administer | |
| BIC, DTG, EVG/c | ↓ BIC, DTG, EVG, COBI expected | Do NOT co-administer | |
| DOR, RPV, ETR, NVP | ↓ NNRTI expected | Do NOT co-administer | |
| MVC | ↓ MVC expected | Do NOT co-administer | |
| All PIs | ↓ PI expected | Do NOT co-administer | |
| EFV | ↓ PI expected | No dose adjustment needed | |
| RAL | Rifapentine 600 mg daily: RAL Cmin ↓ 41% |
Do NOT co-administer RAL with daily rifapentine No dose adjustment needed for once weekly rifapentine and RAL 400 mg twice daily |
|
| EFV | ↔ EFV concentrations | No dose adjustment needed | |
| All other ARVs | No dose adjustment needed | ||
Abbreviations – ARV: antiretroviral; AUC: area under the curve; BIC: bictegravir; COBI: cobicistat; DOR: doravirine; DTG: dolutegravir; EFV: efavirenz; EVG/c: elvitegravir/cobicistat; ETR: etravirine; MVC: maraviroc; NNRTI: non-nucleoside reverse transcriptase inhibitor; NVP: nevirapine; PI: protease inhibitor; RAL: raltegravir; RPV: rilpivirine; TAF: tenofovir alafenamide; TFV: tenofovir
Table 2: Pros and cons of latent tuberculosis infection treatment options9 |
||
| LBTI Treatment | Pros | Cons |
| 9H |
|
|
| 4R |
|
|
| 3HP |
|
|
References:
- Panel on Opportunistic Infections in Adults and Adolescents with HIV. Guidelines for the prevention and treatment of opportunistic infections in adults and adolescents with HIV: recommendations from the Centers for Disease Control and Prevention, the National Institutes of Health, and the HIV Medicine Association of the Infectious Diseases Society of America. Available at http://aidsinfo.nih.gov/contentfiles/guidelines /adult_oi.pdf. Accessed (February 25, 2020) [Pages V3-V22]
- Sterling TR, Scott NA, Miro JM, et al. Three months of weekly rifapentine plus isoniazid for treatment of Mycobacterium tuberculosis infection in HIV co-infected persons. AIDS. 2016;30(10):1607-1615. Available at: http://www.ncbi.nlm.nih.gov/pubmed/26990624.
- Martinson NA, Barnes GL, Moulton LH, et al. New regimens to prevent tuberculosis in adults with HIV infection. N Engl J Med. 2011;365(1):11-20. Available at: http://www.ncbi.nlm.nih.gov/pubmed/21732833.
- Podany AT, Bao Y, Swindells S, et al. Efavirenz pharmacokinetics and pharmacodynamics in HIV-infected persons receiving rifapentine and isoniazid for tuberculosis prevention. Clin Infect Dis. 2015;61(8):1322-1327. Available at:
http://www.ncbi.nlm.nih.gov/pubmed/26082504. - Farenc C, Doroumian S, Cantalloube C, et al. Rifapentine once-weekly dosing effect on efavirenz emtricitabine and tenofovir PKs. Presented at: Conference on Retroviruses and Opportunistic Infections. 2014. Boston, MA. Available at:
http://www.croiconference.org/sessions/rifapentine-once-weekly-dosing-effect-efavirenz-emtricitabine-and-tenofovirpks. - Weiner M, Egelund EF, Engle M, et al. Pharmacokinetic interaction of rifapentine and raltegravir in healthy volunteers. J Antimicrob Chemother. 2014;69(4):1079-1085. Available at http://www.ncbi.nlm.nih.gov/pubmed/24343893.
- Borisov AS, Bamrah Morris S, Njie GJ, et al. Update of recommendations for use of once-weekly isoniazid-rifapentine regimen to treat latent Mycobacterium tuberculosis infection. MMWR Morb Mortal Wkly Rep. 2018;67(25):723-726. Available at: http://www.ncbi.nlm.nih.gov/pubmed/29953429.
- HHS Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in adults and adolescents with HIV. http://www.aidsinfo.nih.gov/ContentFiles/AdultandAdolescentGL.pdf. Updated December 18, 2019. Accessed February 28, 2019.
- Sterling TR, Njie G, Zenner D, et al. Guidelines for the Treatment of Latent Tuberculosis Infection: Recommendations from the National Tuberculosis Controllers Association and CDC, 2020. MMWR Recomm Rep. 2020;69(No. RR-1):1–11. DOI: http://dx.doi.org/10.15585/mmwr.rr6901a1